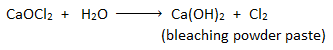
Principle:
Chloroform is prepared in the laboratory by heating ethanol or acetone with aqueous bleaching powder paste. Bleaching powder paste acts as oxidizing, chlorinating and hydrolyzing agent.
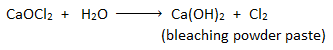
From ethanol :
Step I : Oxidation :

Step II : Chlorination :

Step III : Hydrolysis :
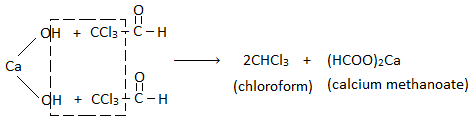
From acetone (propanone) :
Step I : Chlorination :

Step II : Hydrolysis :
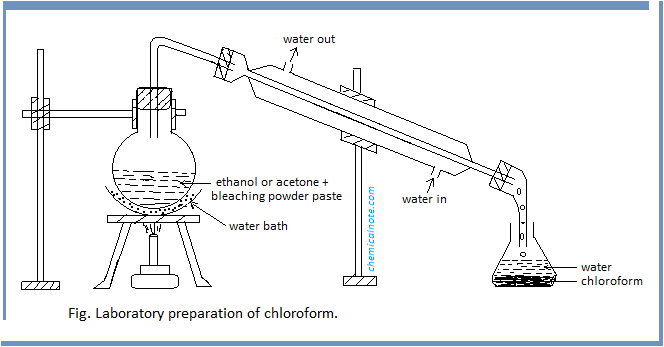
Procedure : First of all, bleaching powder paste is prepared by mixing 100 gm of bleaching powder with 200 ml of water in one liter round bottomed flask and 25 ml of ethanol or acetone is added to it. The flask is heated gently on a water bath until a mixture of chloroform and water distills over, as shown in figure . The mixture from receiver is transferred into a separating funnel and the lower layer of chloroform is separated.